the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
GC Insights: The crystal structures behind mineral properties – a case study of using TotBlocks in an undergraduate optical mineralogy lab
Paige E. dePolo
Spatial thinking represents an ongoing challenge in geoscience education, but concrete manipulatives can bridge this gap by illustrating abstract concepts. In an undergraduate optical mineralogy lab session, TotBlocks were used to illustrate how crystal structures influence properties such as cleavage and pleochroism. More abstracted properties, e.g., extinction angles, were increasingly difficult to illustrate using this tool.
- Article
(2125 KB) - Full-text XML
-
Supplement
(1706 KB) - BibTeX
- EndNote
Spatial thinking and understanding complex 3D structures mark fundamental challenges in geology education (Ishikawa and Kastens, 2005; Liben and Titus, 2012; Woods et al., 2016). These challenges extend to the atomic scale where the crystal structures of minerals are difficult to conceptualize (Dyar et al., 2004). Understanding crystal structures is important because the identifiable features of minerals – e.g., cleavage and pleochroism – ultimately arise from crystal structures and their inherent symmetry (Neumann, 1885). Thus, a more intuitive understanding of these abstract systems is desirable.
Current teaching strategies for visualizing crystal structures include physical manipulatives, e.g., ball-and-stick models, paper polyhedral models, and pre-fabricated hexagonal templates (Rodenbough et al., 2015; Wood et al., 2017; He et al., 1990a, b, 1994; Hollocher, 1997; Mogk, 1997), and virtual manipulatives, e.g., visualization software (Moyer et al., 2002; Extremera et al., 2020). Three-dimensionally printed physical manipulatives can illustrate unit cells in crystallography (Rodenbough et al., 2015), complex structures like DNA (Jittivadhna et al., 2010; Howell et al., 2019), and other chemical principles (Witzel, 2002; Kaliakin et al., 2015; Melaku et al., 2016; Smiar and Mendez, 2016; Geyer, 2017; Lesuer, 2019; Horikoshi, 2020; Melaku and Dabke, 2021).
The TotBlocks project aims to communicate the crystal structures of modular rock-forming chain and sheet silicate minerals (pyroxenes, amphiboles, micas, and clay minerals) through 3D-printed building blocks (Leung and dePolo, 2022a; Fig. 1a). This work investigates the utility of TotBlocks in communicating the relationship between crystal structures and mineral properties.
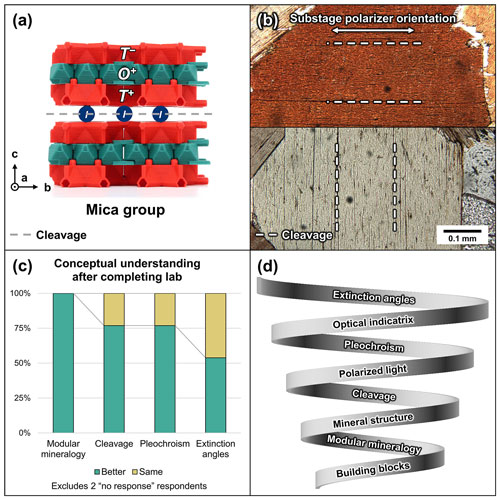
Figure 1(a) The crystal structure of the mica group, illustrated using TotBlocks (Leung and dePolo, 2022a). (b) Example of mineral properties visible under the microscope. Biotite (mica group) displays a perfect basal cleavage on the {001} and displays the strongest pleochroic color when the substage polarizer is parallel to the layers of octahedral modules in (a) (top image). (c) Respondents' understanding of concepts decreased with increasing abstractedness. (d) Proposed spiral learning model for optical mineralogy, based on insights from (c).
A 1 h exercise on modular mineralogy (File S1 in the Supplement) was conducted during the last lab (April 2022) of a second-year optical mineralogy class at Laurentian University (Sudbury, Canada). After a brief introductory lecture, students sequentially built the crystal structures of the mica, pyroxene, and amphibole (super-)groups using TotBlocks. Using these models, students reflected on properties (pleochroism, cleavage, and extinction angles) they had previously discussed during the semester (Fig. 1b). This session was voluntary for students, and attendance was not monitored.
At the end of the exercise, an optional, anonymous feedback survey consisting of four Likert-scale questions and four free-response questions was distributed (File S2 in the Supplement). Students self-assessed whether their understanding of mineral properties was improved by the lab and reflected on what aspects of the lab worked well or could be improved. The data analyzed here (File S3 in the Supplement) were originally collected as teaching feedback. Ethical approval for secondary data usage was granted by the Laurentian University Research Ethics Board (LUREB; no. 6021264).
A total of 15 survey responses were collected. Within these surveys, two respondents (13 %) did not complete the self-assessment section and are tabulated as “no response” for all Likert-scale questions. The terms “better”, “same”, and “worse” were specific responses used in the feedback survey and are employed as such in the following.
No respondents reported a worse understanding of topics at the end of the lab for any Likert-scale question (Fig. 1c). A total of 87 % (13 out of 15) of respondents reported that their understanding of modular mineralogy was better at the end of the lab, and no respondents reported the same level of understanding. The survey responses for understanding pleochroism and cleavage angles were identical, with 67 % (10 out of 15) of respondents reporting that they understood the concepts better and 20 % (3 out of 15) reporting the same level of understanding. The survey responses for the understanding of extinction angles were split more evenly, with 47 % (7 out of 15) of respondents reporting that they understood the concept better and 40 % (6 out of 15) reporting the same level of understanding. Excluding the two no response respondents, 100 % of respondents reported a better understanding of modular mineralogy, 77 % reported a better understanding of cleavage and pleochroism, and 54 % reported a better understanding of extinction angles (Fig. 1c).
All survey participants engaged with the free-response questions, with a general positive consensus observed. Students reported impressions like they “enjoyed the experience” and that the “instructions were clear and the activity very dynamic.”
The use of TotBlocks in this lab setting allowed students to learn mineralogical concepts in alignment with the theory of experiential learning (sensu Kolb and Fry, 1975). Kolb and Fry (1975) conceptualize learning as an iterative, four-stage process that cycles through (1) concrete experience, (2) observations and reflections based upon that experience, (3) analysis of those observations to form abstract conceptualizations, and (4) applying these conceptualizations to new experiences. Through (1) the concrete experience of constructing a mineral structure with TotBlocks, students engage in active and cooperative learning (Smith et al., 2005) and (2) are invited to observe the modularity of different silicate minerals and to reflect on their structural relationships. These reflections provide (3) the abstract foundation for students to then (4) extend these ideas to mineral properties. The process of students using physical manipulatives to solidify their understanding of crystal structures aligns TotBlocks with the educational theory of constructionism (Harel and Papert, 1991).
The structure of the lab exercise additionally followed ideas of spiral learning for mineralogy teaching (Bruner, 1966; Dyar et al., 2004). Students began with the mica structure – the protostructure for other modular rock-forming minerals – and were invited to actively build new concepts through the construction of additional structures. The concepts of cleavage, pleochroism, and extinction angles were introduced in the context of the previously developed ideas. In essence, students began with chemical building blocks, progressed to crystal structures, and then developed further understanding of mineral properties (Fig. 1d).
Using TotBlocks in this classroom setting resulted in some preliminary successes. Students felt the advantages of using physical manipulatives. One student noted “paralleling real-life structures into models” was “easy to understand”, while another reported that “seeing cleavage and extinction in real life” was an aspect of the lab that worked well. Another student observed that “building” was “different in understanding than just being lectured”. These reported experiences illustrate the efficacy of TotBlocks for concretizing abstract ideas of crystal structures for students, similarly to the pattern observed by Fencl and Heunink (2007) in physics classrooms. TotBlocks also allowed students to productively engage in informal cooperative learning (Smith et al., 2005). A student reflected that “having to build the structures as a group of 3–4 people really helped to share concepts and opinions about the question[s]”. This experience illustrates that the use of these manipulatives in the classroom can support peer-to-peer exchange of insights (Boud, 2001; Keerthirathne, 2020). These responses suggest that TotBlocks supported both experiential and cooperative learning in this lab.
Despite these successes, we observed a decrease in the students' understanding of key mineral principles with increasing orders of complexity (Fig. 1c). Although the students' understanding of modular mineralogy improved, fewer students reported similar improvements to their understanding of cleavage and pleochroism. The most challenging concept to impart was extinction angles. This decrease in understanding corresponds to increasing abstractness of concepts – from basic building blocks and crystal structures to polarized light and the optical indicatrix – which is consistent with a spiral learning model (Fig. 1d). This gap in understanding could be addressed by communicating the role of vibration directions in understanding the optical properties of minerals. In particular, a diagram illustrating the relationship between the optical indicatrix and extinction angles might bridge the conceptual gap identified in this case study (for further discussion, see Leung, 2023; File S4 in the Supplement).
We also encountered several practical limitations within the lab, with the most notable being the short time allotted to the exercise. The time restriction was evident for the mineral that concluded the lab, the amphibole structure. Three students noted that building the amphibole structure was confusing, suggesting that additional time on that exercise would have been beneficial. A potential solution would be integrating TotBlocks into multiple lab sessions. Repeated exposure to TotBlocks throughout a term would allow familiarity with physical manipulatives prior to applying them to understanding mineral properties. Additionally, several students noted a need for additional support with the construction instructions of the mineral structures in the lab. They shared thoughts like “I think the building of the structures would be easier with step by step [sic] image[s] (Ikea furniture)” and “it would be helpful to have step by step [sic] instructions with images”. These reflections demonstrate a need for more clarity in task presentation for students (Rosenshine and Stevens, 1986; Rink, 1994). In future classroom applications of TotBlocks, additional building support could be provided to the students through instructional videos (e.g., Leung and dePolo, 2022b). Finally, this study relies on self-reported reflections and lacks an independent metric for assessing learning improvement (i.e., a control group).
Using TotBlocks as concrete manipulatives within experiential, spiral, and cooperative learning frameworks shows potential for improving students' understanding of mineral properties. Incorporating TotBlocks with other representations of crystal structures (e.g., ball-and-stick models and visualization software) in mineralogy classrooms merits further study, particularly in the context of more extended use throughout a course (Tsui and Treagust, 2013).
The full source code and 3D model files for the TotBlocks project (GPLv3 license) can be found on Zenodo: https://doi.org/10.5281/zenodo.5240816 (Leung, 2022).
The Supplement included in this contribution consists of four files: the original lab manual presented to the students (File S1), the survey presented to the students (File S2), the response spreadsheet (File S3), and a revised lab manual reflecting the pedagogical insights gleaned from this study (File S4). The supplement related to this article is available online at: https://doi.org/10.5194/gc-6-125-2023-supplement.
DDVL conceptualized and designed TotBlocks, delivered the lab exercise, collated survey responses, and made the figure. PEdP contextualized TotBlocks in the pedagogical literature and wrote the first draft of this paper. Both the authors designed the lab exercise and survey and discussed and edited the paper.
Derek D. V. Leung holds the copyright for the TotBlocks design files and source code, but these are distributed under a copyleft, open-source license (GPLv3) that is freely available to the public. Additionally, all of the technical design specifications are published in a previous publication (Leung and dePolo, 2022a).
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We thank Sandra Hoy and Lise Carriere (LUREB) for the consultation and assistance in submitting the ethics application, and we thank two anonymous LUREB members for their comments that helped in strengthening the application. We thank Courtney Onstad (Simon Fraser University) for her advice around the language used in ethics assessments. Andrew McDonald (Laurentian University) provided access to the Optical Mineralogy lab session, and Christopher Beckett-Brown and Melissa Barerra assisted. Godfrey Fitton (University of Edinburgh) helped in clarifying our understanding of the optical indicatrix. We thank Geoscience Communication executive editors Sam Illingworth and John Hillier for their advice and guidance around this paper. We also thank David Mogk and Brian Niece for their supportive reviews that helped us clarify the text of this paper and Leslie Almberg for her editorial work in handling the submission.
This paper was edited by Leslie Almberg and reviewed by David Mogk and Brian Niece.
Boud, D.: Introduction: Making the move to peer learning, in: Peer Learning in Higher Education: Learning from & with Each Other, edited by: Boud, D., Cohen, R., and Sampson, J., Routledge, London, UK, 1–17, https://doi.org/10.4324/9781315042565, 2001.
Bruner, J. S.: Toward a Theory of Instruction, Harvard University Press, Cambridge, Massachusetts, USA, ISBN-10 0674897013, ISBN-13 978-0674897014, 1966.
Dyar, M. D., Gunter, M. E., Davis, J. C., and Odell, M. R. L.: Integration of new methods into teaching mineralogy, J. Geosci. Educ., 52, 23–30, 2004.
Extremera, J., Vergara, D., Dávila, L. P., and Rubio, M. P.: Virtual and augmented reality environments to learn the fundamentals of crystallography, Crystals, 10, 456, https://doi.org/10.3390/cryst10060456, 2020.
Fencl, H. and Huenink, A.: An exploration into the use of manipulatives to develop abstract reasoning in an introductory science course, Int. J. Schol. Teach. Learn., 1, 1–15, https://doi.org/10.20429/ijsotl.2007.010215, 2007.
Geyer, M. J.: Using interlocking toy building blocks to assess conceptual understanding in chemistry, J. Chem. Educ., 94, 202–205, https://doi.org/10.1021/acs.jchemed.6b00551, 2017.
Harel, I. and Papert, S. (Eds.): Constructionism, Ablex Publishing, Westport, Connecticut, USA, ISBN-10 0893917869, ISBN-13 978-0893917869, 1991.
He, F.-C., Liu, L.-B., and Li, X.-Y.: Molecular models constructed in an easy way: Part 1. Models of tetrahedron, trigonal bipyramid, octahedron, pentagonal bipyramid, and capped octahedron, J. Chem. Educ., 67, 556–558, https://doi.org/10.1021/ed067p556, 1990a.
He, F.-C., Liu, L.-B., and Li, X.-Y.: Molecular models constructed in an easy way: Part 2. Models constructed by using tetrahedral units as building blocks, J. Chem. Educ., 67, 650–652, https://doi.org/10.1021/ed067p650, 1990b.
He, F.-C., Liu, L.-B., and Li, X.-Y.: Molecular models constructed in an easy way: Part 3. Models constructed by using octahedral units as building blocks, J. Chem. Educ., 71, 734–738, https://doi.org/10.1021/ed071p734, 1994.
Hollocher, K.: Building crystal structure ball models using pre-drilled templates: sheet structures, tridymite, and cristobalite, in: Teaching Mineralogy, edited by: Brady, J. B., Mogk, D. W., and Perkins III, D., Mineralogical Society of America, Washington, D.C., USA, 255–282, ISBN-10 0939950448, ISBN-13 9780939950447 1997.
Horikoshi, R.: Teaching chemistry with LEGO® bricks, Chem. Teach. Int., 3, 239–255, https://doi.org/10.1515/cti-2020-0017, 2020.
Howell, M. E., Booth, C. S., Sikich, S. M., Helikar, T., Roston, R. L., Couch, B. A., and van Dijk, K.: Student understanding of DNA structure-function relationships improves from using 3D learning modules with dynamic 3D print models, Biochem. Mol. Biol. Edu., 47, 303–317, https://doi.org/10.1002/bmb.21234, 2019.
Ishikawa, T. and Kastens, K. A.: Why some students have trouble with maps and other spatial representations, J. Geosci. Educ., 53, 184–197, https://doi.org/10.5408/1089-9995-53.2.184, 2005.
Jittivadhna, K., Ruenwongsa, P., and Panijpan, B.: Beyond textbook illustrations: hand-held models of ordered DNA and protein structures as 3D supplements to enhance student learning of helical biopolymers, Biochem. Mol. Biol. Edu., 38, 359–364, https://doi.org/10.1002/bmb.20427, 2010.
Kaliakin, D. S., Zaari, R. R., and Varganov, S. A.: 3D printed potential and free energy surfaces for teaching fundamental concepts in physical chemistry, J. Chem. Educ., 92, 2106–2112, https://doi.org/10.1021/acs.jchemed.5b00409, 2015.
Keerthirathne, W. K. D.: Peer learning: an overview: Int. J. Sci. Eng. Sci., 4, 1–6, 2020.
Kolb, D. A. and Fry, R.: Towards an applied theory of experiential learning, in: Theories of group processes, edited by: Cooper, C., John Wiley and Sons, New York, 33–57, ISBN 10 0471171174, ISBN 13, 9780471171171, 1975.
Leung, D. D. V.: derekdvleung/totblocks: Totblocks 2022.05 (totblocks-2022.05), Zenodo [code], https://doi.org/10.5281/zenodo.5240816, 2022.
Leung, D. D. V.: “Reply to RC1”, https://doi.org/10.5194/egusphere-2023-294-AC1, 2023.
Leung, D. D. V. and dePolo, P. E.: TotBlocks: exploring the relationships between modular rock-forming minerals with 3D-printed interlocking brick modules, Eur. J. Mineral., 34, 523–538, https://doi.org/10.5194/ejm-34-523-2022, 2022a.
Leung, D. D. V. and dePolo, P. E.: Learning with TotBlocks: Communicating the crystal structures of modular rock-forming minerals with 3D-printed interlocking brick modules, TIB-AV Portal [video], https://doi.org/10.5446/s_1236, 2022b.
Lesuer, R. J.: Incorporating tactile learning into periodic trend analysis using three-dimensional printing, J. Chem. Educ., 96, 285–229, https://doi.org/10.1021/acsami.1c06204, 2019.
Liben, L. S. and Titus, S. J.: The importance of spatial thinking for geoscience education: insights from the crossroads of geoscience and cognitive science, in: Earth and Mind II: A Synthesis of Research on Thinking and Learning in the Geosciences, edited by: Kastens, K. A., and Manduca, C. A., Geological Society of America Special Paper 486, 51–70, https://doi.org/10.1130/2012.2486(10), 2012.
Melaku, S. and Dabke, R. B.: Interlocking toy building blocks as modules for undergraduate introductory and general chemistry classroom teaching, J. Chem. Educ., 98, 2465–2470, https://doi.org/10.1021/acs.jchemed.1c00001, 2021.
Melaku, S., Schreck, J. O., Griffin, K., and Dabke, R. B.: Interlocking toy building blocks as hands-on learning modules for Blind and Visually Impaired Chemistry Students, J. Chem. Educ., 93, 1049–1055, 2016.
Mogk, D. W.: Directed-discovery of crystal structures using ball and stick models, in: Teaching Mineralogy, edited by: Brady, J. B., Mogk, D. W., and Perkins III, D., Mineralogical Society of America, Washington, D.C., USA, 283–290, 1997.
Moyer, P. S., Bolyard, J. J., and Spikell, M. A.: What are virtual manipulatives, Teach. Child. Math., 8, 372–377, https://doi.org/10.5951/TCM.8.6.0372, 2002.
Neumann, F.: Vorlesungen über die Theorie der Elasticität der festen Körper und des Lichtäthers, edited by: Meyer, O. E., B. G. Teubner-Verlag, Leipzig, Germany, ISBN-10 0270359516, ISBN-13 978-0270359510, 1885.
Rink, J. E.: Task Presentation in Pedagogy, Quest, 46, 270–280, https://doi.org/10.1080/00336297.1994.10484126, 1994.
Rodenbough, P. P., Vanti, W. B., and Chan, S.-W.: 3D-printing crystallographic unit cells for learning materials science and engineering, J. Chem. Educ., 92, 1960–1962, https://doi.org/10.1021/acs.jchemed.5b00597, 2015.
Rosenshine, B. and Stevens, R.: Teaching functions, in: Handbook of research on teaching, 3rd Edn., edited by: Wittrock, M., Macmillan, New York, USA, 376–391, ISBN-10 0029003105, ISBN-13 978-0029003107, 1986.
Smiar, K. and Mendez. J. D.: Creating and using interactive, 3D-printed models to improve student comprehension of the Bohr model of the atom, bond polarity, and hybridization, J. Chem. Educ., 93, 1591–1594, https://doi.org/10.1021/acs.jchemed.6b00297, 2016.
Smith, K. A., Sheppard, S. D., Johnson, D. W., and Johnson, R. T.: Pedagogies of engagement: Classroom-based practices, J. Eng. Ed., 94, 87–101, 2005.
Tsui, C.-Y. and Treagust, D. F.: Introduction to multiple representations: their importance in biology and biological education, in: Multiple Representation in Biological Education, edited by: Treagust, D. F. and Tsui, C.-Y., Springer, 3–18, https://doi.org/10.1007/978-94-007-4192-8_1, 2013.
Witzel, J. E.: Lego Stoichiometry, J. Chem. Educ., 79, 352A, https://doi.org/10.1021/ed079p352, 2002.
Wood, P. A., Sarjeant, A. A., Bruno, I. J., Macrae, C. F., Maynard-Casely, H. E., and Towler, M.: The next dimension of structural science communication: simple 3D printing directly from a crystal structure, CrystEngComm, 19, 690, https://doi.org/10.1039/c6ce02412b, 2017.
Woods, T. L., Reed, S., Hsi, S., Woods, J. A., and Woods, M. R.: Pilot study using the augmented reality sandbox to teach topographic maps and surficial processes in introductory geology labs, J. Geosci. Educ., 64, 199–214, https://doi.org/10.5408/15-135.1, 2016.





